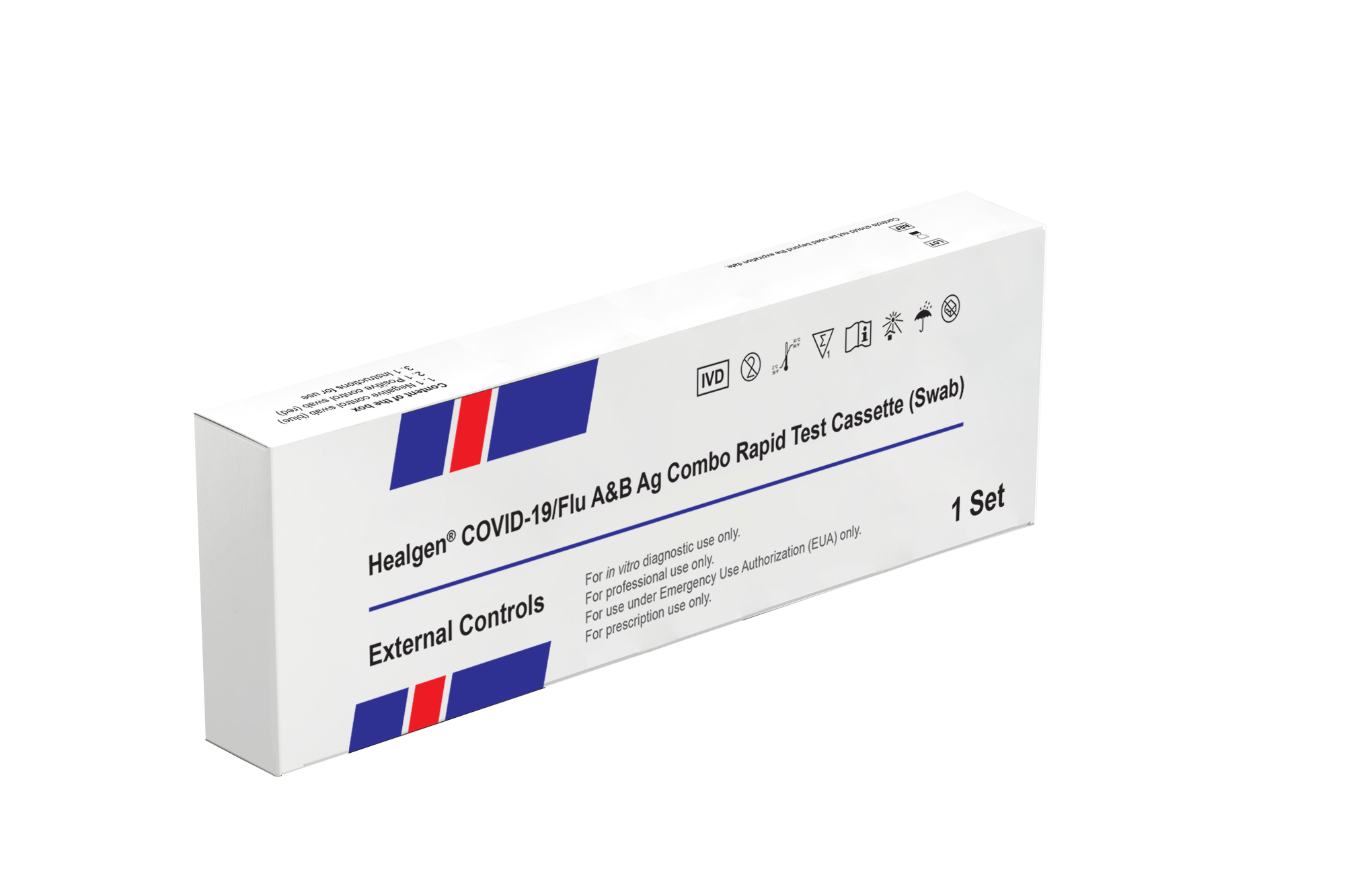
Rapid COVID-19 Flu A/B External Controls
$4.85
Positive Negative External Controls
Ready-to-use external control kit for use with the Healgen rapid COVID-19 Flu A/B antigen combination test. This kit is to verify proper test procedure and performance of the COVID-19 Flu A/B combo rapid test cassette. A CLIA number is required to purchase this test. For in vitro diagnosis and prescription use only.
A single box contains one negative and one positive control.
Call 800.742.4909 to place an order.
-
Description
Description
Positive, Negative External Controls
Ready-to-use external control kit for use with the Healgen rapid COVID-19 Flu A/B antigen combination test. This kit is to verify proper test procedure and performance of the COVID-19 Flu A/B combo rapid test cassette.
The positive control swabs are composed of a SARS-CoV-2 recombinant antigen, Influenza A recombinant antigen and Influenza B recombinant antigen extract dried onto a swab, with a red shaft, containing 0.05% Proclin 300 as a preservative. The negative control swabs are composed of negative control buffer dried onto a swab, with a blue shaft, containing 0.05% Proclin 300 as a preservative.
- Always use disposable gloves and protective gear clothing while administering an external control.
- Do not eat, drink, smoke in the area where an external control is being conducted.
- All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
- Control swabs and test device should be discarded in proper biohazard containers.
- Use the swabs within 2 hours of opening the sealed pouch.
Each box includes
- 1, Negative control swab (blue)
- 1, Positive control swab (red)
- Instructions for use
-
Specifications
Additional information
Weight .01 lbs Dimensions 6 × 3 × 1.5 in -
EUA Statement
EUA Statement
Authorized Laboratories:
Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This product is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
The Healgen® COVID-19/Flu A&B Ag Combo Rapid test Cassette (Swab) has not been FDA cleared or approved but has been authorized by FDA under an EUA for use by authorized laboratories.
This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens.The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
-
Product Documentation
Product Documentation
-
Reviews (0)
Be the first to review “Rapid COVID-19 Flu A/B External Controls” Cancel reply


Reviews
There are no reviews yet.